Molecular Cardiology
Explore the molecular cardiology research programs and labs below.
Paul Burridge, PhDInvestigating the pharmacogenomics of chemotherapy-induced toxicity and electrophysiological disorders using human induced pluripotent stem cells.
Investigating the pharmacogenomics of chemotherapy-induced toxicity and electrophysiological disorders using human induced pluripotent stem cells.
The Burridge Lab works in the fields of pharmacogenomics (precision medicine), cardio-oncology, cardiovascular disease modeling, regenerative medicine, cancer, and cultivated meat, using induced pluripotent stem cells (iPSCs) as our model.
We specialize in large-scale hiPSC projects that required reprogramming, sequencing, editing, differentiating, and phenotyping hundreds of hiPSC lines.
Ongoing projects include:
- Studying the role of the genome in complex disease traits, understanding how single genetic variants modulate drug efficacy and toxicity, predicting which patients will experience successful therapy or suffer adverse drug responses, and discovering new drugs to overcome these complications.
- Developing new tools to reprogram, culture, and differentiate iPSC in an efficient and cost-effective manner to improve the use, scale, and ubiquity of these across all areas of research.
- Studying the genomics of arrhythmia and sudden cardiac death and devising precision medicine-led drug discovery.
- The applications of iPSC and direct reprogramming in regenerative medicine, repairing the heart after a heart attack, or ameliorating the effects of heart failure.
- How heritable (germline) variation influences cancer progression, oncology drug efficacy, and acquisition of resistance.
- The applications of iPSC-derived cells for cultivated meat.
Contact Us
Searle 8-450 320 E. Superior St.Chicago, IL 60611United States
paul.burridge@northwestern.edu
See the faculty profile for Paul W. Burridge, PhD, or visit the Burridge Lab website to learn more.
Mesut Eren, PhDStudying the molecular physiology of the plasminogen activation system.
Primarily, Eren is interested in investigating the role of plasminogen activator inhibitor type-1 (PAI-1) in senescence and aging of various tissues including cardiovascular, renal and pulmonary tissues as well as cardiac fibrosis, heart failure and hair loss.
In order to study how PAI-1 affects the molecular mechanisms of these pathophysiologies, we developed novel mouse models, including the mice line that expresses the identical loss-of-function (LOF) mutant form of PAI-1 present in Berne Amish kindred and the line that lacks extracellular PAI-1 activity. Furthermore, we developed new monoclonal antibodies to detect and quantify this LOF mutant form of PAI-1 to quantify and test if it has any physiological and/or pathophysiological roles. Additionally, we have generated mice lines that have PAI-1 deficiency in cardiomyocytes and endothelial cells to explore the transcriptomics and metabolimcs changes in cardiovascular aging, cardiac fibrosis and heart failure with preserved ejection fraction (HFpEF).
Moreover, we have started an novel project that involves studying the role of PAI-1 in age dependent hearing loss using our mouse models described above in addition to the transgenic mice that express a functionally stable variant of human PAI-1.
See the faculty profile for Mesut Eren, PhD, to learn more.
Al George, MDInvestigating the structure, function, pharmacology and molecular genetics of ion channels and channelopathies.

Ion channels are ubiquitous membrane proteins that serve a variety of important physiological functions, provide targets for many types of pharmacological agents and are encoded by genes that can be the basis for inherited diseases affecting the heart, skeletal muscle and nervous system.
Our research program is focused on the structure, function, pharmacology and molecular genetics of ion channels. We were the first to determine the functional consequences of a human cardiac sodium channel mutation associated with an inherited cardiac arrhythmia. The lab has elucidated the functional and molecular consequences of several brain sodium channel mutations that cause various familial epilepsies and an inherited form of migraine. These finding have motivated pharmacological studies designed to find compounds that suppress aberrant functional behaviors caused by mutations.
See the faculty profile for Al George, MD, to learn more.
Songwang Hou, PhDStudying phospholidpid functions in cells and anti-phosphatidylethanolamine antibody-resulted diseases in patients.
Hou's current research focus on two main aspects. One is the basic research of phospholidpid functions in cells, which includes their localization in membrane networks, the mechanisms of controlling phospholipid asymmetry and the pathological effects upon the defects. The another is the mechanism research of anti-phosphatidylethanolamine antibody resulted diseases in patients.
See the faculty profile of Songwang Hou, PhD, to learn more.
Sadiya S. Khan, MD, MScExamining the epidemology of and risk for heart failure.
Our studies include population-based cohorts, large electronic health record data analysis, and -OMICS (epigenomics, genomics, and proteomics) to perform mechanistic studies to enhance risk prediction and identify novel therapeutic agents for prevention and treatment approaches.
See the faculty profile for Sadiya S. Khan, MD, MSc, to learn more.
Francis Klocke, MDFocusing on coronary circulation and cardiac magnetic resonance imaging.
See the faculty profile for Francis Klocke, MD, to learn more.
Tsutomu Kume, PhDFocused on cardiovascular development and disease with an emphasis on the formation of the vascular system that is composed of blood and lymphatic vessels; we study the cellular and molecular mechanisms that control neovascularization in physiological and pathological processes using various mouse models.
Focused on cardiovascular development and disease with an emphasis on the formation of the vascular system that is composed of blood and lymphatic vessels; we study the cellular and molecular mechanisms that control neovascularization in physiological and pathological processes using various mouse models.
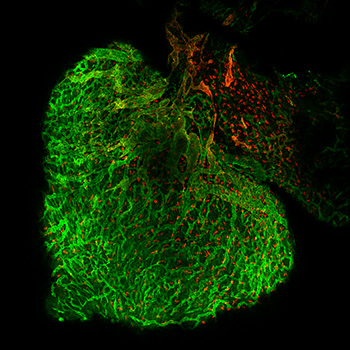
The Kume Laboratory is recognized for studies highlighting the role of a family of transcription factors termed Forkhead/Fox in diverse biological processes. Key observations include studies elucidating the role of Foxc1 and Foxc2 as (1) factors that serve as essential regulators of cardiovascular development, (2) key factors of angiogenesis and lymphangiogenesis, and (3) important regulators of the anterior eye segment. These research efforts have spawned novel areas of investigation with therapeutic implications for numerous biological systems, including the biology of the cardiovascular systems.
Studies in the lab have recently extended toward the area of the organ-specific vasculature, and we have established a research team to investigate mechanisms of tissue repair and regeneration using a variety of mouse models including ischemia-reperfusion injury.
See the faculty profile for Tsutomu Kume, PhD, to learn more. Daniel Lee, MDFocusing on both the technical development and clinical application of cardiovascular magnetic resonance imaging.
Lee's research laboratory focuses on both the technical development and clinical application of cardiovascular magnetic resonance imaging (CMR).
Viability Imaging
Initial research efforts focused on validating this technique’s ability to measure the extent of myocardial infarction and myocardial viability. Current investigations explore its use as a tool to measure, salvage, and predict remodeling following myocardial infarction, identify patients at risk for ventricular arrhythmias, and gauge the effect of experimental therapies on infarct size.
Myocardial Perfusion Imaging
Our research has demonstrated that first-pass magnetic resonance perfusion imaging has advantages over traditional radionuclide imaging for detecting modest reductions in myocardial blood flow and subtle differences between endocardial and epicardial flow. Investigators in our laboratory are currently developing techniques to measure absolute myocardial blood flow (in mL/min/g), which may allow more precise determination of blood flow limitations. These new techniques are and will be employed to characterize myocardial blood flow in patients with coronary artery disease, microvascular dysfunction, heart failure, and in studies of drug and stem cell therapy.
CMR Core Laboratory
CMR noninvasively obtains high-resolution images with excellent depiction of blood, infarct, and myocardial borders resulting in high reproducibility of measurements. Not surprisingly, CMR is increasingly being used in clinical trials to quantify left ventricular volumes, mass and infarct size.
See the faculty profile for Daniel C. Lee, MD, to learn more.
Elizabeth M. McNally, MD, PhDIdentifying and studying mechanisms of mutations that cause genetic disease.
Mutations in cytoskeletal and sarcomere proteins cause heart failure and muscle weakness. We use novel computational methods to analyze whole genome sequencing from humans to identify novel genes and alleles linked to human disease. We model these mutations in human cells using induced pluripotent stem cell technologies to determine how these mutations act. We are defining gene-gene interactions using genome analysis, genome editing and quantitative trait locus mapping.
See the faculty profile for Elizabeth M. McNally, MD, PhD, or visit the McNally Lab website to learn more.
Ed Thorp, PhDInvestigating the cell and molecular biology of the cardiovascular system in steady state versus during inflammation.
Investigating the cell and molecular biology of the cardiovascular system in steady state versus during inflammation.
See the faculty profile for Ed Thorp, PhD, or visit Thorp Lab website to learn more.
Doug Vaughan, MDInvestigating the role of the plasminogen activator system in cardiovascular disease.
Investigating the role of the plasminogen activator system in cardiovascular disease.
Vaughan directs a multidisciplinary research group focused on investigating the role of the plasminogen activator system in cardiovascular disease. Active experimental programs are underway at the molecular and cellular level in animals and in humans. Transgenic and knockout mice are used in a variety of studies designed to explore the tissue-specific expression of PAI-1 in vivo and the role of the fibrinolytic system in vascular disease and tissue remodeling.
See the faculty profile for Douglas E. Vaughan, MD, to learn more.
Lisa D. Wilsbacher, MD, PhDStudying the roles of G protein-coupled receptors in heart development and in the setting of adult heart disease.
Studying the roles of G protein-coupled receptors in heart development and in the setting of adult heart disease.
The goal of our research is to define key signaling pathways in cardiac development and cardiomyocyte maintenance in the setting of pathological stress. Currently, the laboratory investigates the G protein-coupled receptor sphingosine-1-phosphate receptor 1 (S1P1) and its unexpected role in cardiomyocyte proliferation and cardiac development. We aim to identify the signaling mechanisms that underlie these cardiac developmental effects and to investigate whether S1P1 signaling contributes to cardiac remodeling in the adult heart.
See the faculty profile for Lisa Wilsbacher, MD, PhD, and visit the Wilsbacher Lab site to learn more.
Ming Zhao, PhDDeveloping diagnostic markers and investigates pathogenic mechanisms of human diseases based on changes in cellular membranes.
Major research areas in the Zhao Lab include:
- Apoptosis imaging technology development. Programmed cell death (apoptosis) plays a significant role in degenerative diseases. There is currently no clinical tool for assessing apoptosis in pathological conditions. Our research focuses on the development of optimal agents that combine sophisticated binding activities and favorable clearance kinetics for clinical translation.<.li>
- Assessing systemic toxicity in anticancer therapies. The outcome of chemotherapies hinges on the balance between tumor toxicity and patient tolerance. With the ability to noninvasively detect tissue apoptosis, we propose to assess anticancer therapies in a whole-body approach by monitoring tumor cell killing simultaneously with systemic tissue injury in response to chemotherapeutic agents. This is a transformative approach in oncology in terms of optimizing therapies on an individualized basis.
- Detecting myocardial injury in ischemic heart disease. Non-infarct myocardial injury in ischemic heart disease is of particular interest because this type of cardiac injury is not well understood in terms of its pathophysiological characteristics and its roles in long-term adverse cardiac events. Our research in this area focuses on the diagnosis of non-infarct myocardial injury, which in turn, will help address a significant gap in identifying patients at risk.
- Investigating the pathogenesis of antiphospholipid syndromes. The presence of circulating antibodies against phosphatidylethanolamine (PE) is positively correlated with clinical manifestations of antiphospholipid syndromes. However, the underlying pathogenic mechanism of anti-PE autoimmunity remains unknown. We have a major interest in investigating the cellular susceptibility to PE-binding agents, which in turn, will shed light on the potential pathogenic mechanism of aPE.
See the faculty profile for Ming Zhao, PhD, to learn more.